Which of the Following Best Describes an Isotope
How is an isotope different from the standard form of a chemical element. Which best describes the subject of a story.
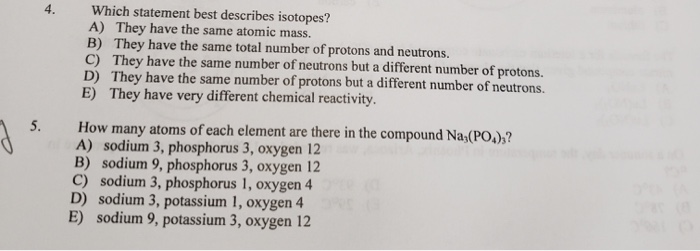
Solved Which Statement Best Describes Isotopes A They Have Chegg Com
Which one of the following statements best describes the stability of the iron isotope.
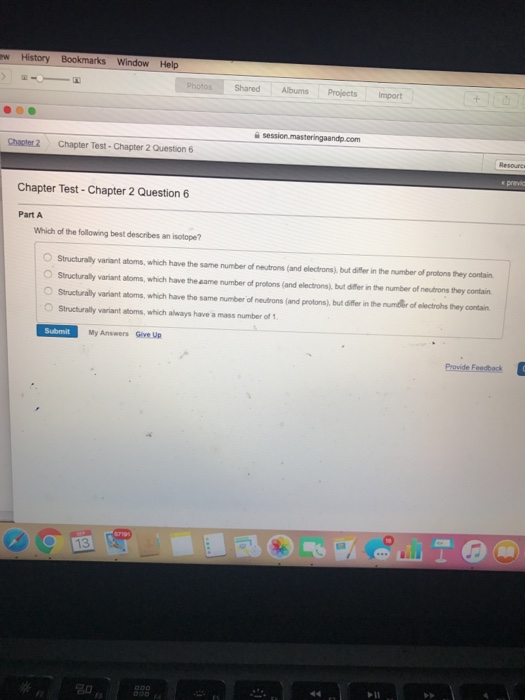
. D They have the same number of protons but a different number of neutrons. Which of the following best defines covalent bonds. D Isotopes occur only in the heavier elements.
A All the isotopes of an element have the same number of neutrons. What describes a radioisotope. All three isotopes of hydrogen have identical.
As the atomic number is same these elements have the same position on the periodic table. Uranium-235 and uranium-238 occur naturally in the Earths crust. For example ³H an isotope of hydrogen ¹⁴C an isotope of carbon ⁸⁷Rb an isotope of rubidium etc.
In the field of chemistry isotopes can be described as elements that have the same atomic number but different mass number. They have different numbers of electrons D. B They have the same total number of protons and neutrons.
Which of the following isotopes is not a radioisotope. An atom that has bonded with another atom to form a molecule. The bond formed when shared electrons occupy a single orbital common to both atoms within a molecule.
A A labelled sample of a known biosynthetic precursor is fed to a culture. Isotopes differ from each other because their nucleon number or mass number is different. Part A Which of the following best describes an isotope.
Isotopes are the two or more forms of the same element that differ in the number of neutrons but the number of protons and electrons remain the same. Which of the following best describes an isotope. Carbon 12 and Carbon 14 are both isotopes of carbon one with 6 neutrons and one with 8 neutrons both with 6 protons.
An atom that has more or fewer neutrons than protons. Brainpop carbon dating DRAFT. An isotope of an element that is radioactive.
Consider the plot of binding energy per nucleon versus the nucleon number A. Which of the following statements best describes how Atom X and Atom Y are related. Both have long half-lives.
For example carbon-12 is an isotope of carbon with a mass number of 12. Carbon-12 is a stable isotope while carbon-14 is a radioactive isotope radioisotope. An enzyme inhibitor is added to block one of the stages in the.
C They have the same number of neutrons but a different number of protons. Structurally variant atoms which have the same number of neutrons and electrons but differ in the number of protons they contain. Which of the following best describes the nucleus of an atom of carbon-14.
An atom that has gained or lost electrons and has an electrical charge. Select the correct statement about isotopes. Which of the following best describes isotopes.
This isotope of hydrogen contains 1 proton 1 electron and 2 neutrons. The atomic weight not mass of a chemical element is calculated considering the percentage concentatration of natural isotopes. Isotopes have been found as variations of atoms.
Which of the following can be determined from the frequency of a. An atom that has changed into another atom through loss or gain of protons. Which of the following best describes an isotope.
Hence all of the above options are correct. Structurally variant atoms which have the same number of protons and electrons but differ in the number of neutrons they contain. A Carbon-13 b Carbon-14 c Tritium d Sulphur-35 Question 2.
This isotope of hydrogen contains 1 proton 1 electron and no neutrons. Which statement best describes isotopes. An isotope is named after the element and the mass number of its atoms.
An element has an atomic number of 16. Some elements have radioactive isotopes. They have different numbers of protons B.
What best describes the weight average of atomic mass on an element. B Isotopes of the same element have the same atomic number but differ in their atomic masses. C All the isotopes of an element are radioactive.
It can also be noted that this isotope of hydrogen is radioactive. A They have the same atomic mass. Atoms can be considered the basic building blocks of matter.
Which of the following statements best describes an intermediate trapping experiment. They have different electron shells. Atom Y has 9 protons 9 neutrons and 9 electrons.
This isotope of hydrogen contains 1 proton 1 electron and 1 neutron. A This isotope has the most stable nucleus because a minimum amount of work is needed to separate this nucleus into its constituent protons and neutrons. They have different numbers of neutrons C.
Atom X has 9 protons 10 neutrons and 9 electrons. Structurally variant atoms which have the same number of protons and electrons but differ in the number of neutrons they contain.

Chapter 2 The Structure Of The Atom Learning Outcomes Sub Atomic Particles Protons Neutrons And Electrons Isotopes Structure Of The Atom Ppt Download

Solved Question 31 Which Statement Best Describes An Isotope When Atoms From Different Elements Have The Atoms That Whose Same Physical And Chemical Properites Nucleii Contaln Equal Numbers Of Protons An Atom Of

Solved Which Of The Following Best Describes An Isotope Chegg Com
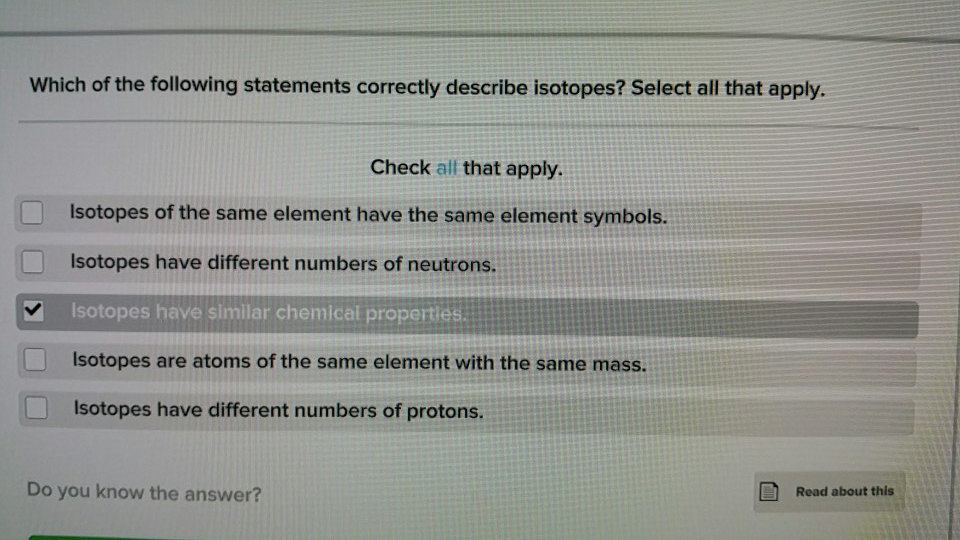
Solved Which Of The Following Statements Correctly Describe Chegg Com
No comments for "Which of the Following Best Describes an Isotope"
Post a Comment